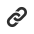Company
Corederm Co., Ltd. specializes in conducting skin clinical trials for cosmetics, household goods, over-the-counter drugs, health functional foods, and beauty devices.
IRB
Institutional Review Board, IRB
The Institutional Review Board (IRB) are standing committees that are independently set up and operated by testing laboratories. They continuously review the clinical trial protocol and the methods used to obtain written consent and related information from the subjects, so that their rights, safety, and welfare can be protected.
The IRB audit conducted by Corederm Dermatological Research Institute, Inc. follows basic ethical principles such as the Helsinki Declaration, the Nuremberg Code, and the ICH GCP Guidelines to protect the human rights and welfare of the subjects.
Corederm’s IRB examination is carried out according to the standard work guidelines prepared by KGCP and domestic related laws and regulations.